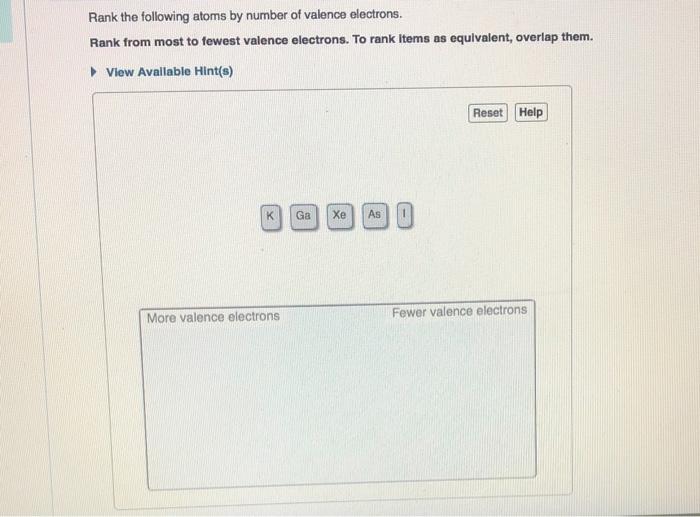
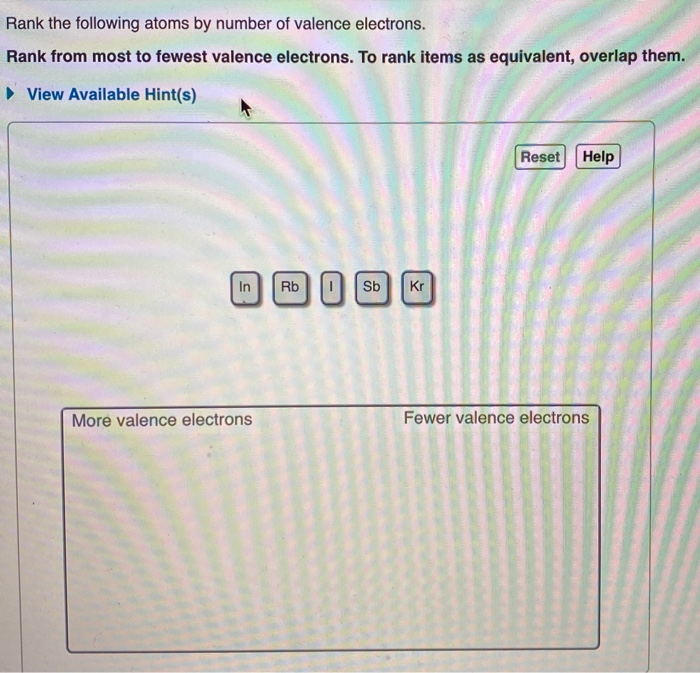
Rank the Following Atoms by Number of Valence Electrons
Rank from most to fewest valence electrons. An element behaves as a non-metal when the number of valence electrons in its atom is more than four.
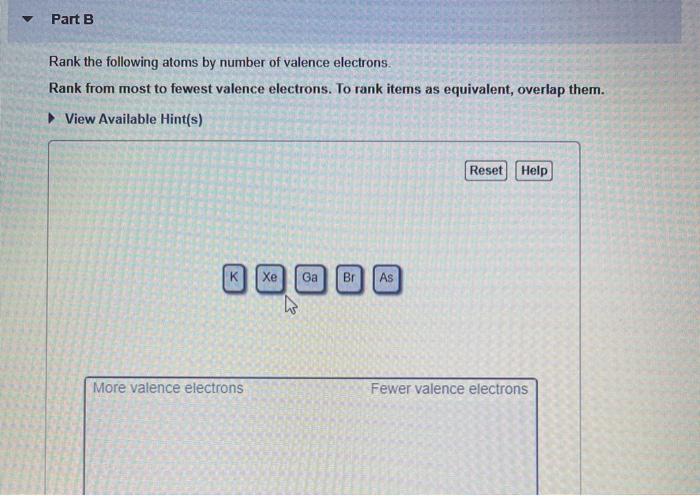
Solved Part B Rank The Following Atoms By Number Of Valence Chegg Com
View Available Hints Reset Help More valence electrons Fewer valence electrons.

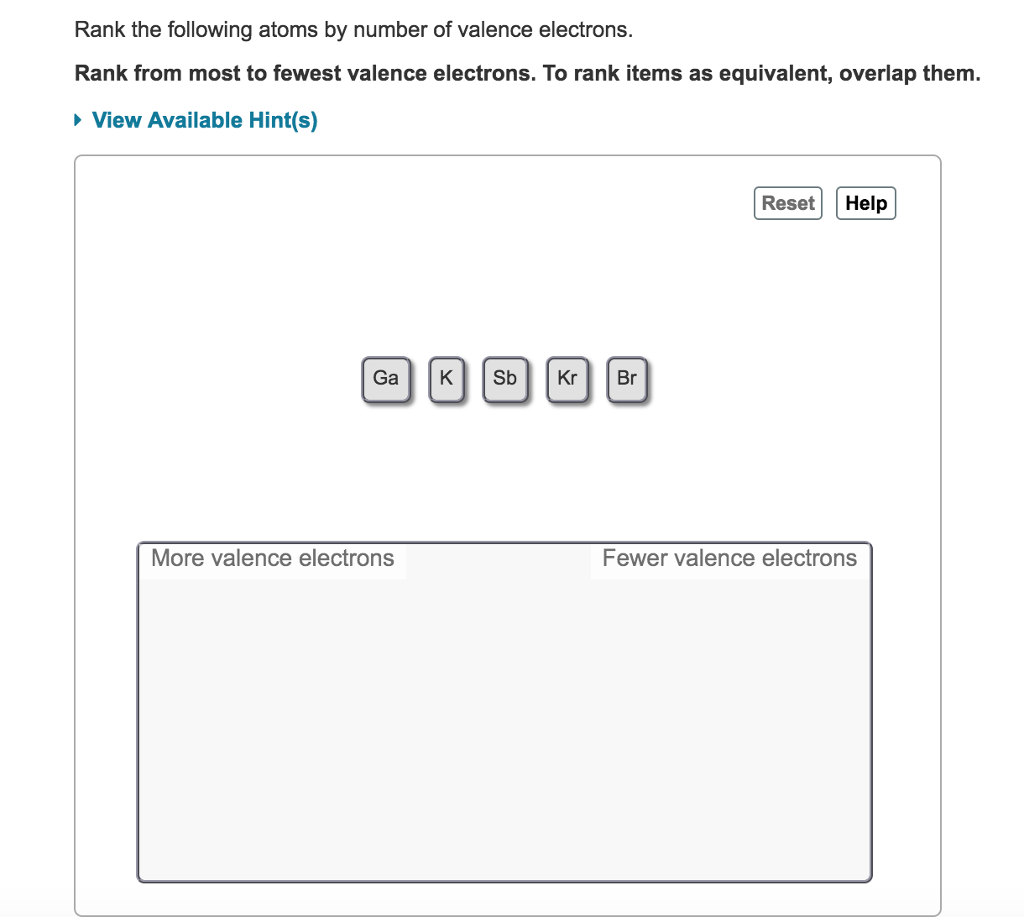
. In Einsteins interpretation of the photoelectric effect the maximum kinetic energy of the ejected electrons is given in terms of the frequency ν and the work function Φ by a. Kr Sb Ga I K. Rank the following atoms by number of valence electrons.
Up to 256 cash back Rank the following atoms by number of valence electrons. Kr 5s² 4d¹⁰ 5p¹ - 3 valence electrons. He 2 s 2 2 p 1 B has 3 valence electrons.
Ge 32 protons 70. Rank the following atoms by number of valence electrons. Arrange the following atoms in order of increasing effective nuclear charge experienced by the electrons in the n3 electron shell.
Carbon silicon and germanium are semiconductor elements and these have precisely four valence electrons in their atoms. B 039 100 points Consider the following molecular. To rank items as equivalent overlap them.
1 2 m e υ 2 h ν - b. K Mg P Rh and Ti. Ba 2I - IBaI.
Electronic configuration of Number of valence electrons 7. Ca 20 protons 42 mass number 20 electrons 22 neutrons. Oxygen is in group 6.
To rank items as equivalent overlap them. Electronic configuration of Number of valence electrons 2. Kr 5s² 4d¹⁰ 5p⁶ - 8 valence electrons.
Electronic configuration of Number of valence electrons 1. K 2O - KOO. The non-metals are a bad conductor of electricity.
Rank the following atoms by number of valence electrons. Kr 5s² 4d¹⁰ 5p⁵ - 7 valence electrons. For neutral atoms the number of valence electrons is equal to the atoms main group number.
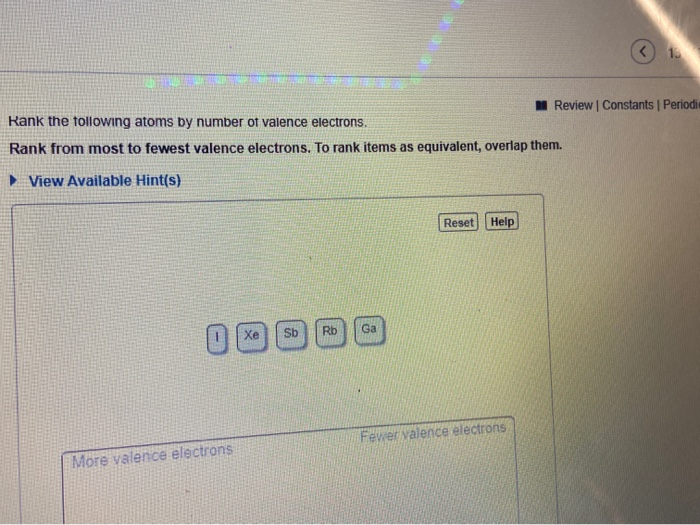
Trends are based on Coulombs law which mathematically relates several characteristics of an elements. So in ranking it would be Xe Sb In Rb. See the answer See the answer done loading.
The electron - dot notation for B is therefore. Sb has 5 valence electrons In has 3 Rb has 1 valence electron and Xe has 8. Please tell me how this is done thank you.
To rank items as equivalent overlap them. 1 2 m e υ 2 hv Φ d. To rank items as equivalent overlap them.
Complete the chart indicating the element symbol and the number of protons neutrons and electrons in each atom. Kr 5s² 4d¹⁰ 5p³ - 5 valence electrons. Rank the following atoms by their number of valence electrons from most to fewest.
Periodic trends predict differences between elemental characteristics as you move across the periodic table. Such elements or materials are called as the semiconductor. 1 2 m e υ 2 hv Φ e.
Drag the appropriate labels to their respective targets. This problem has been solved. For example carbon is in group 4 and has 4 valence electrons.
Atomic size decreases from. Draw the Lewus structure for the following atoms and assign the correct number of valence electrons and correct charges. None of these c.
Rank from most to fewest valence electrons. Atomic size measured the distance between the nucleus of an atom and the outermost non-valence electrons of the atom. Kr 5s¹ - 1 valence electron.
K By signing up youll get. The number of protons in the ion is the biggest determinate of the size when electron number is constant. Rank from most to fewest valence electrons.
Electronic configuration of Number of valence electrons 5. Rank from smallest to largest. Xe I Sb In Rb.
Rank from most to fewest valence electrons. The number of ejected electrons increases. Rank the following atoms and ions Li Be 2 He H B 3 in order of decreasing size 1.
Rank from most to fewest valence electrons. The main group number for an element can be found from its column on the periodic table. Electronic configuration of Number of valence electrons 7.
If two protons and two neutrons are removed from the nucleus of an oxygen-16 atom a nucleus of which element remains. 1 2 m e υ 2 v h Φ Φ. To rank items as equivalent overlap them.
Are most elements metallic or nonmetallic.
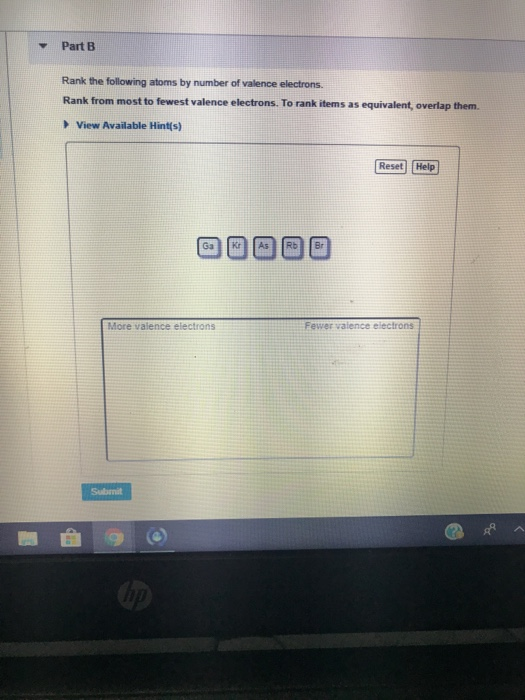
Solved Part B Rank The Following Atoms By Number Of Valence Chegg Com

What Is The Aufbau Principle Science Chemistry Chemistry Classroom Chemistry Lessons
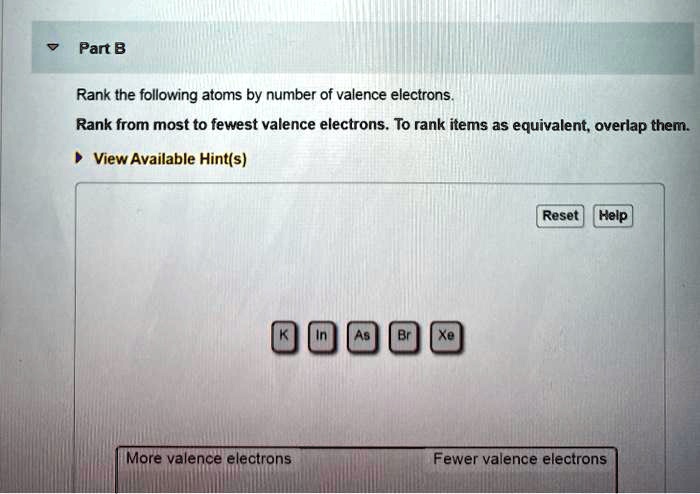
Solved Part B Rank The Following Atoms By Number Of Valence Electrons Rank From Most To Fewest Valence Electrons To Rank Items As Equivalent Overlap Them View Available Hint S Reset Help More Valence
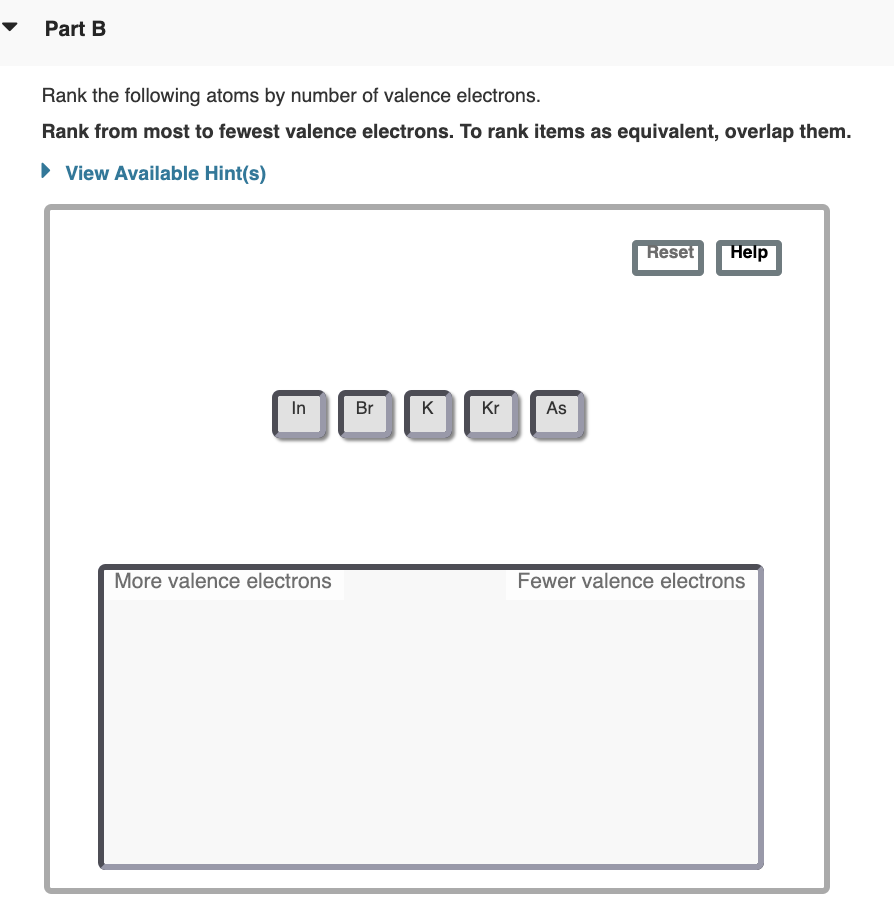
Solved Part B Rank The Following Atoms By Number Of Valence Chegg Com
Solved Rank The Following Atoms By Number Of Valence Chegg Com
Solved Rank The Following Atoms By Number Of Valence Chegg Com
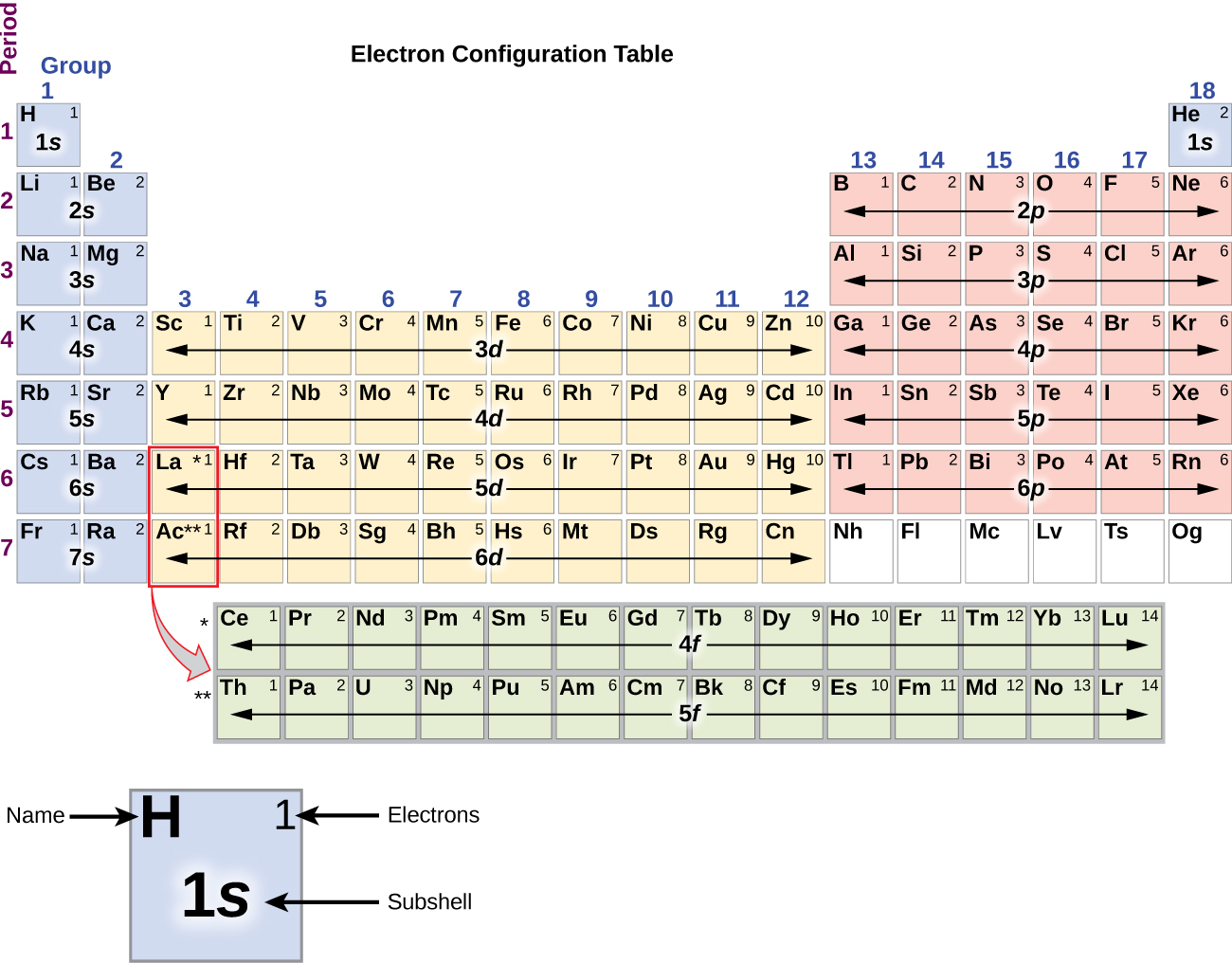
3 1 Electron Configurations Chemistry Libretexts

So2 Lewis Structure How To Draw The Lewis Structure For So2 Sulfur Dioxide Chemistry Lessons Molecular Geometry Chemistry Worksheets

Mcat Question Of The Day Can You Get It Correct Check Here Link In Bio Https Www Motivatemd Com Mcat Question Of Th Question Of The Day Mcat Mcat Prep
Solved Review Constants Periodi Rank The Following Atoms Chegg Com
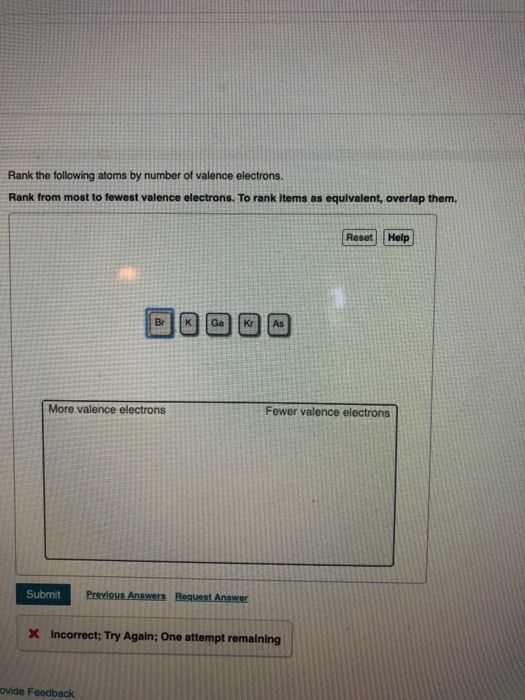
Solved Rank The Following Atoms By Number Of Valence Chegg Com

Lewis Structures Chemistry Lessons Chemistry Classroom Teaching Chemistry

Ch 8 Atomic Electron Configuration And Chemical Periodicity
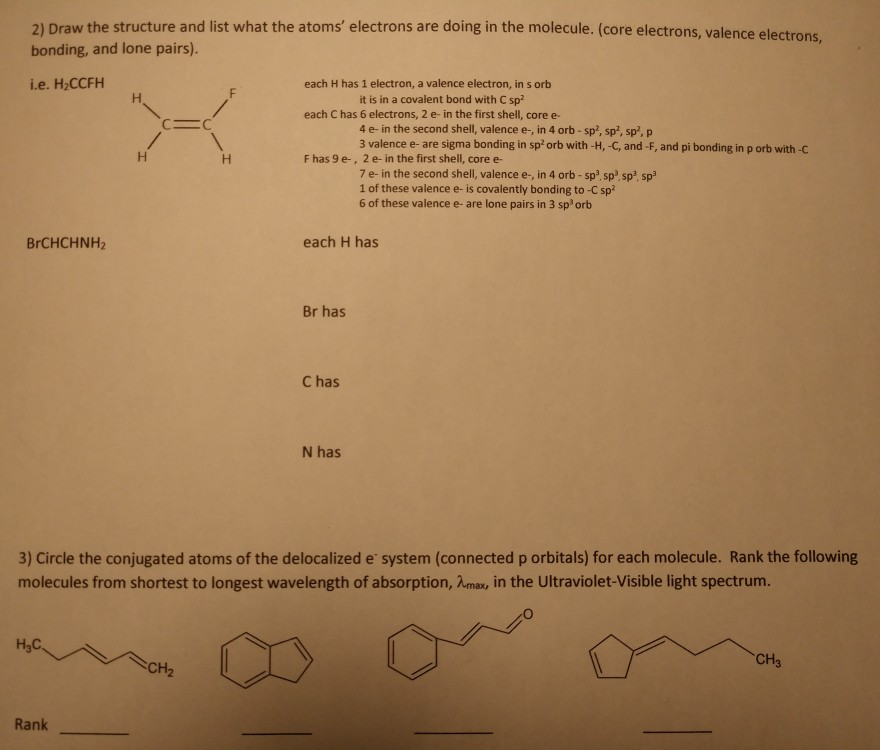
Solved 21 Draw The Structure And List What The Atoms Chegg Com
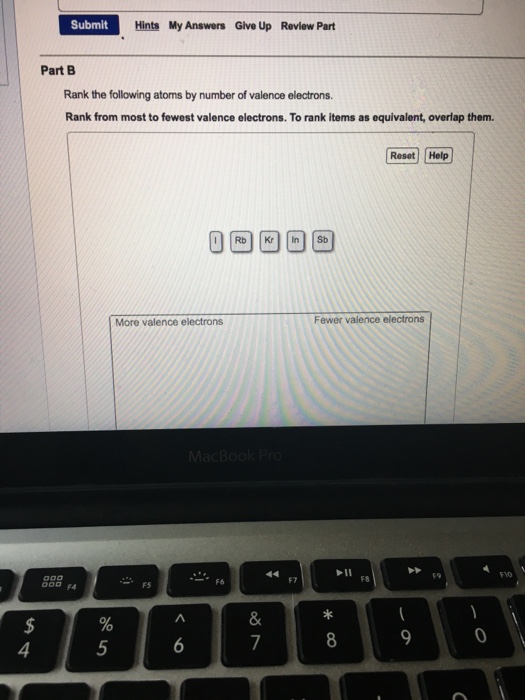
Solved Rank The Following Atoms By Number Of Valence Chegg Com

Electron Configurations Practice Khan Academy

How Many Valence Electrons Does Carbon Have Perfect Atom Electron Configuration Electrons Covalent Bonding
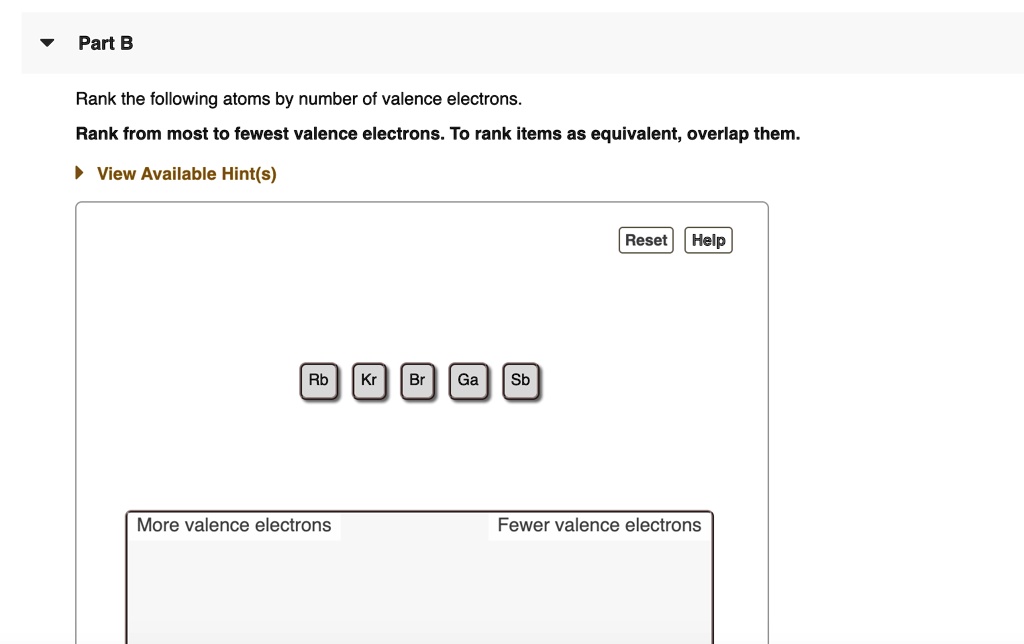
Solved Part B Rank The Following Atoms By Number Of Valence Electrons Rank From Most To Fewest Valence Electrons To Rank Items As Equivalent Overlap Them View Available Hint S Reset Help Rb Ga
Solved Rank The Following Atoms By Number Of Valence Chegg Com
Comments
Post a Comment